Molecule With Both Ionic and Covalent Bond
A basic definition of an ionic compound is that they are molecules that consist of charged ions. Therefore whatever bonds it makes are ionic.

Ionic Vs Covalent Which Is Which And How To Tell Them Apart
When electrons are shared and molecules form covalent bonds result.

. Compounds that consist primarily of elements other than carbon and hydrogen are called inorganic compounds. What compounds have both ionic and covalent bonds. Covalent bonds are the attractive forces between the positively charged nuclei of the bonded atoms and one or more.
Calcium carbonate is another example of a compound with both ionic and covalent bonds. Covalent bonding involves the sharing of electrons between. Is made up of ionic bond between metal and non metal.
NH42CO3NH4ClNH42SO4 are some compounds that have both ionic and covalent bondsAmmonium compounds normally have bothFor example in ammonium carbonate N-H. The two types of bonds are ionic bonds and covalent bonds. Not quite sure what youre asking.
On the other hand covalent. Ionic ones contain a metal and a nonmetal and covalent compounds are two non-metals. It explains ionic and covalent bonding and basic naming instructions and examples.
Also any compound that contains the ammonium NH4. This form of chemical bonding is typical in carbon-based compounds. Here calcium acts as the cation with the.
The pair of electrons which. Print both pages and make your copies front and back to have a one page foldable. Calcium carbonate is another example of a compound with both ionic and covalent bonds.
A molecule or compound is made when two or more atoms form a chemical bond linking them together. Covalent Bonds in Binary Compounds Ionic Bonds Form when electrons are transferred between atoms. Lab Manual Introductory Chemistry A Green.
Covalent bonding is the sharing of electrons between atoms. The best guide to the covalent or ionic character of a bond is to consider the types of atoms involved and their relative positions in the periodic table. Form between a metal and a non-metal.
Bonds between two nonmetals are. Here calcium acts as the cation with the carbonate species as the anion. For example sodium Na a metal and chloride Cl a nonmetal form an ionic bond to make NaCl.
Ionic bonds usually occur between metal and nonmetal ions. The sharing of electrons between atoms is referred to as a covalent bond. These ions have opposite both negative and positive charges.
Likewise the Na and Cl atoms in NaCl have an electronegativity difference of 21 and the Mn and I atoms in MnI 2 have a difference of 10 yet both of these substances form ionic compounds. By definition an ionic bond is between a metal and a nonmetal and a covalent bond is between 2. Ionic and Covalent Bonding.
Is made up of ionic bond between metal and non metal. This type of bonding occurs between two atoms of the same element or of elements close to each other in the. Cl contains both an iconic and covalent bond.
The ammonium ion is polyatomic which means it forms ionic salts. They include both covalent and ionic compounds. Ionic And Covalent Bonding Lab Conclusion YouTube.
These species share an ionic bond while the carbon and oxygen. There is a couple different ways to determine if a bond is ionic or covalent. 3 potassium nitrate.
There are primarily two forms of bonding that an atom can participate in. Lab 1 Ionic And Molecular Compounds Doc Google Accounts. However the bond between N.
A dead giveaway the compound contains both bonds is when it has a metal cation bonded to an anion that only contains nonmetals. In a covalent bond the atoms.
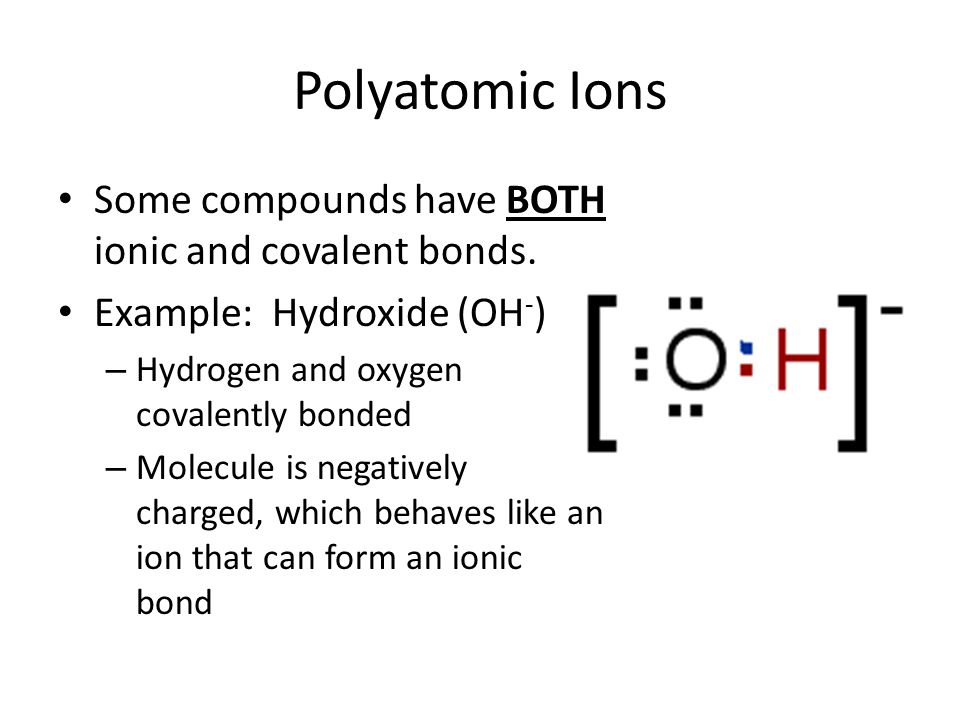
Ionic And Covalent Bonding Ppt Download

What Are Some Compounds That Contain Both Ionic And Covalent Bonds Quora

Ionic Bonds Vs Covalent Bonds Chemtalk
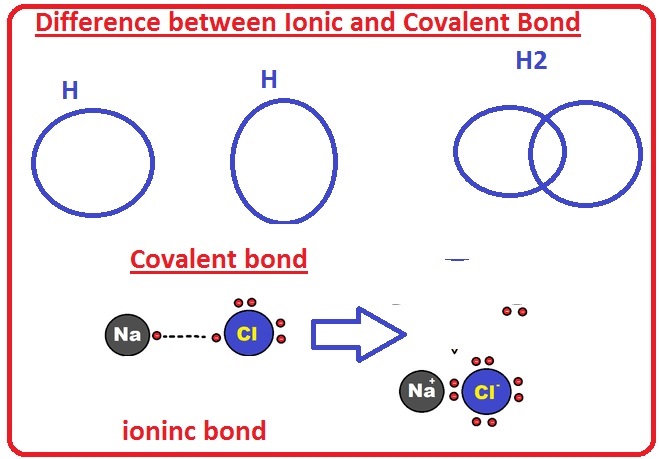
Difference Between Ionic And Covalent Bond The Engineering Knowledge

Comments
Post a Comment